Retrosynthesis#
Retrosynthesis is a fundamental task in drug discovery. Given a target molecule, the goal of retrosynthesis is to identify a set of reactants that can produce the target.
In this example, we will show how to predict retrosynthesis using G2Gs framework. G2Gs first identifies the reaction centers, i.e., bonds generated in the product. Based on the reaction centers, the product is broken into several synthons and each synthon is translated to a reactant.
Prepare the Dataset#
We use the standard USPTO50k dataset. This dataset contains 50k molecules and their synthesis pathways.
First, let’s download and load the dataset. This may take a while.
There are two modes to load the dataset. The reaction mode loads the dataset as
(reactants, product) pairs, which is used for center identification. The synthon
mode loads the dataset as (reactant, synthon) pairs, which is used for synthon
completion.
from torchdrug import data, datasets, utils
reaction_dataset = datasets.USPTO50k("~/molecule-datasets/",
atom_feature="center_identification",
kekulize=True)
synthon_dataset = datasets.USPTO50k("~/molecule-datasets/", as_synthon=True,
atom_feature="synthon_completion",
kekulize=True)
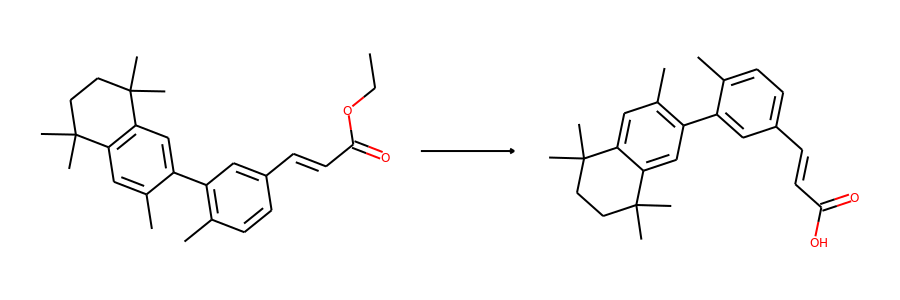
Then we visualize some samples from the dataset. For the reaction dataset, we can
split reactant and product graphs into individual molecules using
connected_components().
Note USPTO50k ignores all non-target products, so there is only one product on
the right hand side.
from torchdrug.utils import plot
for i in range(2):
sample = reaction_dataset[i]
reactant, product = sample["graph"]
reactants = reactant.connected_components()[0]
products = product.connected_components()[0]
plot.reaction(reactants, products)

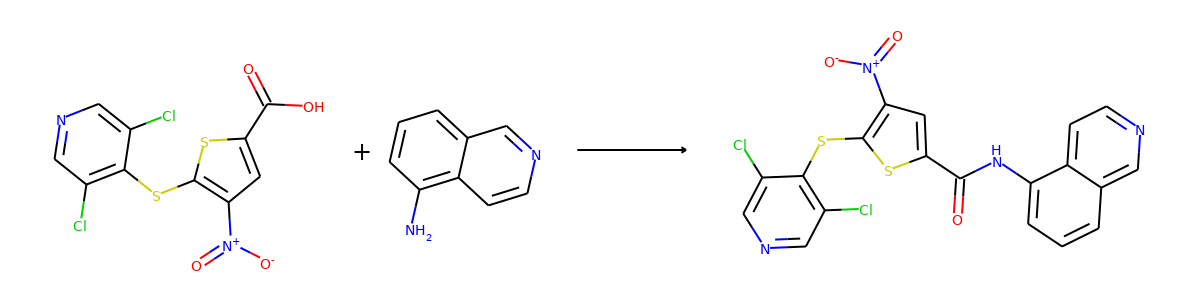
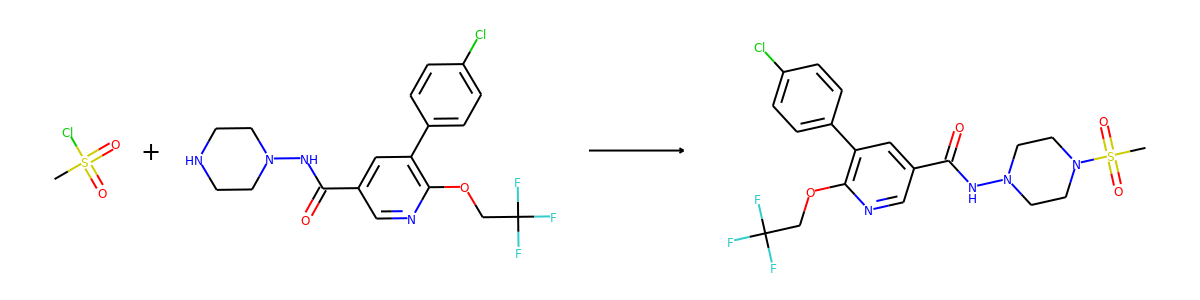
Here are the corresponding samples in the synthon dataset.
for i in range(3):
sample = synthon_dataset[i]
reactant, synthon = sample["graph"]
plot.reaction([reactant], [synthon])
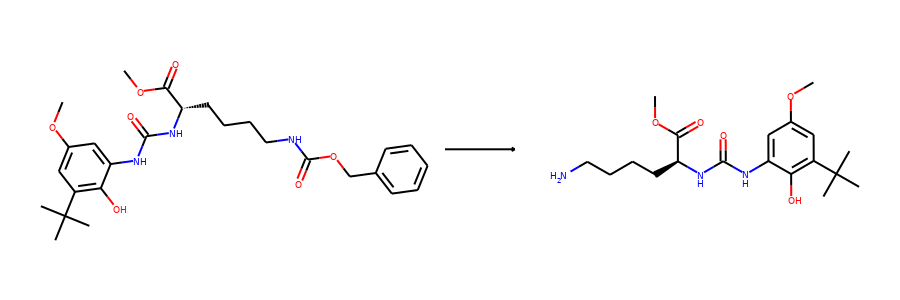
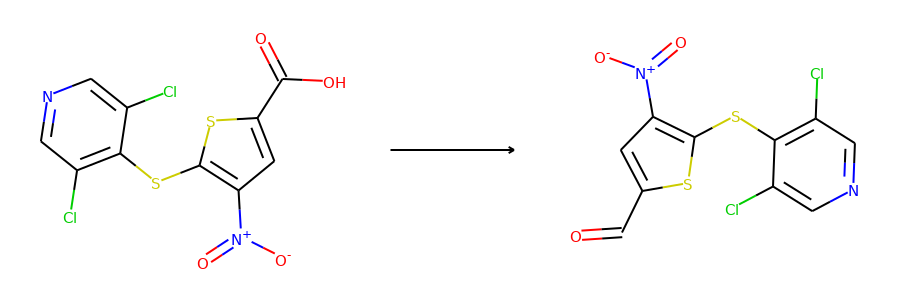
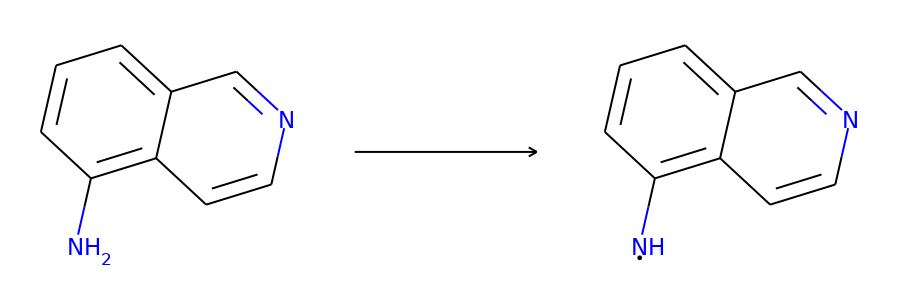
To ensure the same split is used by both datasets, we can set the random seed
before calling split().
import torch
torch.manual_seed(1)
reaction_train, reaction_valid, reaction_test = reaction_dataset.split()
torch.manual_seed(1)
synthon_train, synthon_valid, synthon_test = synthon_dataset.split()
Center Identification#
Now we define our model. We use a Relational Graph Convolutional Network (RGCN) as our representation model, and wrap it for the center identification task. Note other graph representation learning models can also be used here.
from torchdrug import core, models, tasks
reaction_model = models.RGCN(input_dim=reaction_dataset.node_feature_dim,
hidden_dims=[256, 256, 256, 256, 256, 256],
num_relation=reaction_dataset.num_bond_type,
concat_hidden=True)
reaction_task = tasks.CenterIdentification(reaction_model,
feature=("graph", "atom", "bond"))
reaction_optimizer = torch.optim.Adam(reaction_task.parameters(), lr=1e-3)
reaction_solver = core.Engine(reaction_task, reaction_train, reaction_valid,
reaction_test, reaction_optimizer,
gpus=[0], batch_size=128)
reaction_solver.train(num_epoch=50)
reaction_solver.evaluate("valid")
reaction_solver.save("g2gs_reaction_model.pth")
The evaluation result on the validation set may look like
accuracy: 0.836367
We can show some predictions from our model. For diversity, we collect samples from 4 different reaction types.
batch = []
reaction_set = set()
for sample in reaction_valid:
if sample["reaction"] not in reaction_set:
reaction_set.add(sample["reaction"])
batch.append(sample)
if len(batch) == 4:
break
batch = data.graph_collate(batch)
batch = utils.cuda(batch)
result = reaction_task.predict_synthon(batch)
The following code visualizes the ground truths as well as our predictions on the samples. We use blue for ground truths, red for wrong predictions, and purple for correct predictions.
def atoms_and_bonds(molecule, reaction_center):
is_reaction_atom = (molecule.atom_map > 0) & \
(molecule.atom_map.unsqueeze(-1) == \
reaction_center.unsqueeze(0)).any(dim=-1)
node_in, node_out = molecule.edge_list.t()[:2]
edge_map = molecule.atom_map[molecule.edge_list[:, :2]]
is_reaction_bond = (edge_map > 0).all(dim=-1) & \
(edge_map == reaction_center.unsqueeze(0)).all(dim=-1)
atoms = is_reaction_atom.nonzero().flatten().tolist()
bonds = is_reaction_bond[node_in < node_out].nonzero().flatten().tolist()
return atoms, bonds
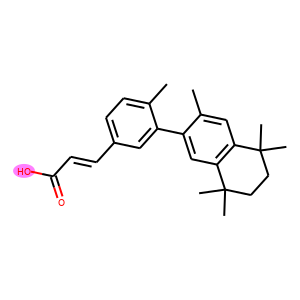
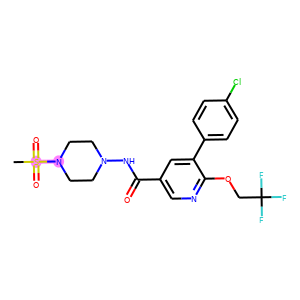
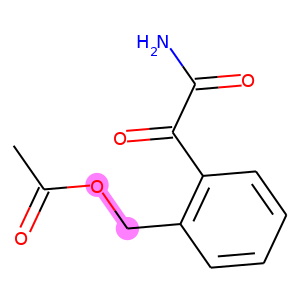
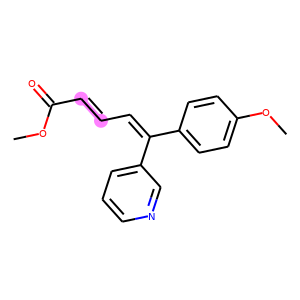
products = batch["graph"][1]
reaction_centers = result["reaction_center"]
for i, product in enumerate(products):
true_atoms, true_bonds = atoms_and_bonds(product, product.reaction_center)
true_atoms, true_bonds = set(true_atoms), set(true_bonds)
pred_atoms, pred_bonds = atoms_and_bonds(product, reaction_centers[i])
pred_atoms, pred_bonds = set(pred_atoms), set(pred_bonds)
overlap_atoms = true_atoms.intersection(pred_atoms)
overlap_bonds = true_bonds.intersection(pred_bonds)
atoms = true_atoms.union(pred_atoms)
bonds = true_bonds.union(pred_bonds)
red = (1, 0.5, 0.5)
blue = (0.5, 0.5, 1)
purple = (1, 0.5, 1)
atom_colors = {}
bond_colors = {}
for atom in atoms:
if atom in overlap_atoms:
atom_colors[atom] = purple
elif atom in pred_atoms:
atom_colors[atom] = red
else:
atom_colors[atom] = blue
for bond in bonds:
if bond in overlap_bonds:
bond_colors[bond] = purple
elif bond in pred_bonds:
bond_colors[bond] = red
else:
bond_colors[bond] = blue
plot.highlight(product, atoms, bonds, atom_colors, bond_colors)
Synthon Completion#
Similarly, we train a synthon completion model on the synthon dataset.
synthon_model = models.RGCN(input_dim=synthon_dataset.node_feature_dim,
hidden_dims=[256, 256, 256, 256, 256, 256],
num_relation=synthon_dataset.num_bond_type,
concat_hidden=True)
synthon_task = tasks.SynthonCompletion(synthon_model, feature=("graph",))
synthon_optimizer = torch.optim.Adam(synthon_task.parameters(), lr=1e-3)
synthon_solver = core.Engine(synthon_task, synthon_train, synthon_valid,
synthon_test, synthon_optimizer,
gpus=[0], batch_size=128)
synthon_solver.train(num_epoch=10)
synthon_solver.evaluate("valid")
synthon_solver.save("g2gs_synthon_model.pth")
We may obtain some results like
bond accuracy: 0.983013
node in accuracy: 0.967535
node out accuracy: 0.892999
stop accuracy: 0.929348
total accuracy: 0.844374
We then perform beam search to generate reactant candidates.
batch = []
reaction_set = set()
for sample in synthon_valid:
if sample["reaction"] not in reaction_set:
reaction_set.add(sample["reaction"])
batch.append(sample)
if len(batch) == 4:
break
batch = data.graph_collate(batch)
batch = utils.cuda(batch)
reactants, synthons = batch["graph"]
reactants = reactants.ion_to_molecule()
predictions = synthon_task.predict_reactant(batch, num_beam=10, max_prediction=5)
synthon_id = -1
i = 0
titles = []
graphs = []
for prediction in predictions:
if synthon_id != prediction.synthon_id:
synthon_id = prediction.synthon_id.item()
i = 0
graphs.append(reactants[synthon_id])
titles.append("Truth %d" % synthon_id)
i += 1
graphs.append(prediction)
if reactants[synthon_id] == prediction:
titles.append("Prediction %d-%d, Correct!" % (synthon_id, i))
else:
titles.append("Prediction %d-%d" % (synthon_id, i))
# reset attributes so that pack can work properly
mols = [graph.to_molecule() for graph in graphs]
graphs = data.PackedMolecule.from_molecule(mols)
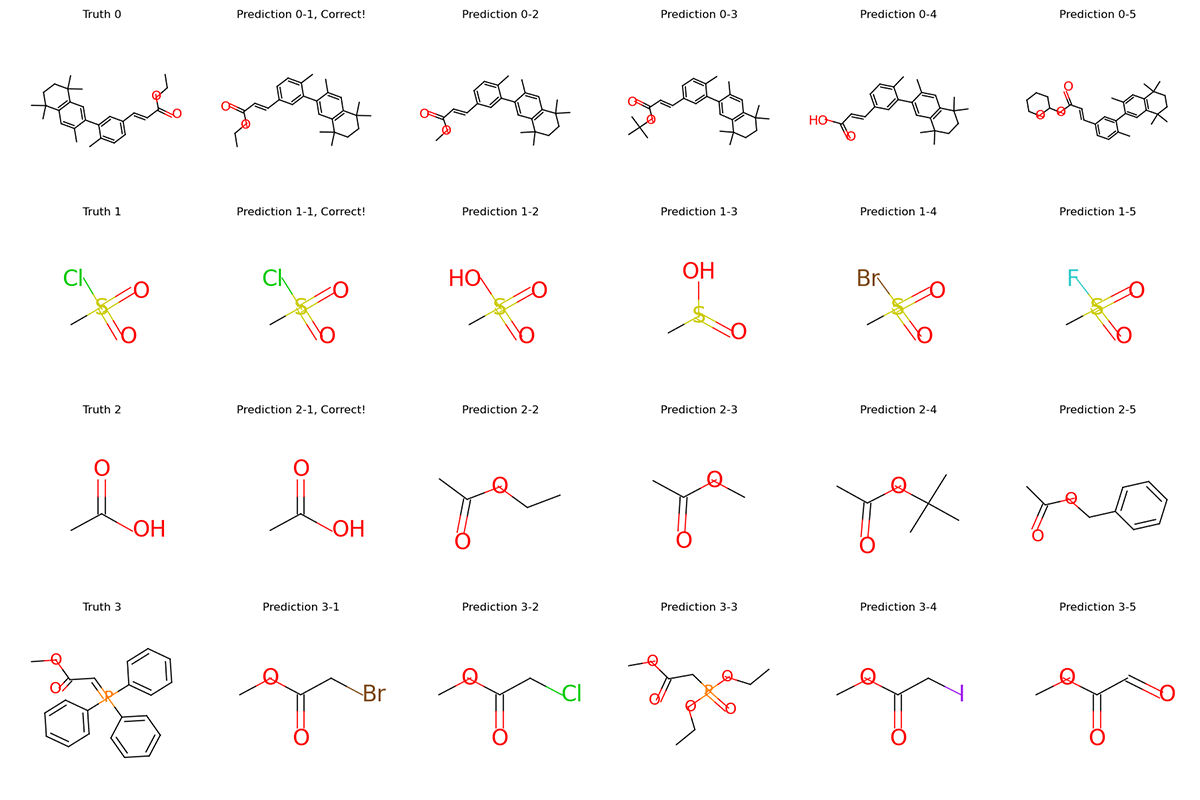
graphs.visualize(titles, save_file="uspto50k_synthon_valid.png", num_col=6)
For each row in the visualization, the first molecule corresponds to the ground truth. The rest molecules are candidates from beam search, ordered by their log likelihood. We can see that our model can recall ground truth in top-5 predictions for most samples.
Retrosynthesis#
Given the trained models, we can combine them into an end2end pipeline for retrosynthesis. This is done by wrapping the two sub tasks with a retrosynthesis task.
Note if you never declare the solvers for reaction_task and synthon_task,
you need to manually call their preprocess() method before combining them into a
pipeline.
# reaction_task.preprocess(reaction_train, None, None)
# synthon_task.preprocess(synthon_train, None, None)
task = tasks.Retrosynthesis(reaction_task, synthon_task, center_topk=2,
num_synthon_beam=5, max_prediction=10)
The pipeline will perform beam search over all possible combinations between the predictions from two sub tasks. For demonstration, we use a small beam size and only evaluate on a subset of the validation set. Note the results will be better if we give more budget to the beam search.
from torch.utils import data as torch_data
lengths = [len(reaction_valid) // 10,
len(reaction_valid) - len(reaction_valid) // 10]
reaction_valid_small = torch_data.random_split(reaction_valid, lengths)[0]
optimizer = torch.optim.Adam(task.parameters(), lr=1e-3)
solver = core.Engine(task, reaction_train, reaction_valid_small, reaction_test,
optimizer, gpus=[0], batch_size=32)
To load parameters two sub tasks, we just load each checkpoint. Note load_optimizer
should be set to False to avoid conflicts.
solver.load("g2gs_reaction_model.pth", load_optimizer=False)
solver.load("g2gs_synthon_model.pth", load_optimizer=False)
solver.evaluate("valid")
The accuracy for retrosynthesis may be close to the following.
top-1 accuracy: 0.47541
top-3 accuracy: 0.741803
top-5 accuracy: 0.827869
top-10 accuracy: 0.879098
Here are the top-1 predictions for samples in the validation set.
batch = []
reaction_set = set()
for sample in reaction_valid:
if sample["reaction"] not in reaction_set:
reaction_set.add(sample["reaction"])
batch.append(sample)
if len(batch) == 4:
break
batch = data.graph_collate(batch)
batch = utils.cuda(batch)
predictions, num_prediction = task.predict(batch)
products = batch["graph"][1]
top1_index = num_prediction.cumsum(0) - num_prediction
for i in range(len(products)):
reactant = predictions[top1_index[i]].connected_components()[0]
product = products[i].connected_components()[0]
plot.reaction(reactant, product)